Latest News
Posted On: Aug 26, 2019
TANZANIA MEDICINES AND MEDICAL DEVICES AUTHORITY

ISO 9001: 2015 CERTIFIED
PUBLIC NOTICE
22nd August, 2019
FALSIFIED “COLD CAP” CAPSULES IN THE TANZANIAN MARKET
- Tanzania Medicines and Medical Devices Authority (TMDA) is an Executive Agency under the Ministry of Health, Community Development, Gender, Elderly and Children, which is responsible for regulating quality, safety and effectiveness of medicines, medical devices and diagnostics.
- Through the surveillance system, TMDA has encountered a falsified medicine in the market, named as “Cold cap” in form of capsules, which is purported to be manufactured in India without declaration of the details of manufacturer.
- The appearance of the falsified “Cold cap” differs significantly from that of the genuine “Coldcap”, which is manufactured by Regal Pharmaceuticals Ltd, Nairobi-Kenya and registered by TMDA.
The key features which distinguish the falsified medicine from the genuine one are as described below:-
NO. FALSIFIED MEDICINE - COLD CAP GENUINE MEDICINE - COLDCAP 3.1 Appearance of the outer package (box) 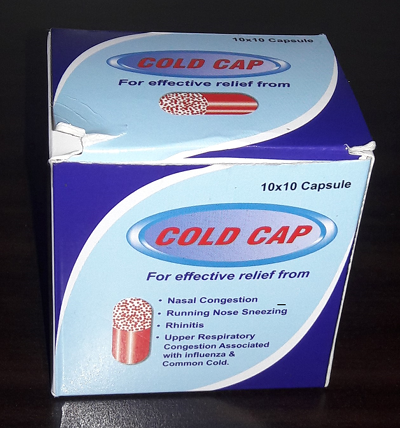
- Has patterns of blue and light blue colors, which are separated from each other by a thick line of white color.
- Has a pictorial presentation of a capsule, which has red color on one side and a red dot on the other side. There is no any letter or a word written on it.
- The name ‘Cold cap’ is presented as two separate words.
- No name and address of manufacturer.
Appearance of the outer package (box) 
- Has a pattern of light pink color, which is surrounded by blue color.
- Has a pictorial presentation of a pink capsule with a letter R in a circle and words COLDCAP ORIGINAL there under, which are in red color.
- The name ‘Coldcap’ is presented as a single word.
- Manufacturer name is declared as Regal Pharmaceuticals Ltd, Nairobi Kenya
3.2 Appearance of the capsule:- 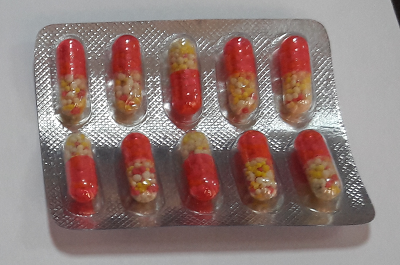
- The bottom side of the capsule is clear and transparent and the top side is pink in color and opaque.
- Filled with round granules of yellow, white and pink colors, which are easily visible through the transparent side of capsules.
Appearance of the capsule:- 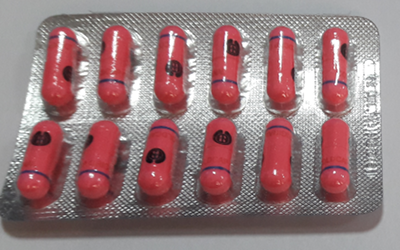
- All sides of the capsule are pink in color and opaque.
- Filled with white colored powder, which can only be viewed after opening the two parts of a capsule.
- With this notice, the general public is urged to scrutinize the features presented in the packaging boxes and capsules carefully in order to avoid purchase or use of the stated falsified medicine.
- Individuals, retailers and wholesalers who unknowingly possess the falsified “Cold Cap” capsules are required to take them back to where they have been purchased from or surrender them to any of the TMDA zone offices in Dar es Salaam, Arusha, Mwanza, Mbeya, Mtwara, Simiyu, Tabora and Dodoma or any nearby district or regional hospital.
- The public is further encouraged to report any suspected substandard or falsified medicine, medical devices and diagnostics or any person who engage in clandestine manufacturing and illegal marketing of the regulated products.
- TMDA will continue to strengthen regulation of medicines, medical devices and diagnostics in order to protect public health.
Acting Director General,
Tanzania Medicines and Medical Devices Authority (TMDA),
Mwanza Street, Block T, Plot No.6,
P. O. Box 1253, Dodoma Or
P. O. Box 77150, Dar es Salaam
Phone: +255 22 2452108/2450512/2450751
Fax: +255 22 2450793
Hotline: 0800110084

