Procedures for Marketing Authorization
This part of our website includes information for marketing authorization holders and other industry stakeholders. Products for which medicinal claims are made or which contain substances likely to have effects on the body are considered as medicines, and will therefore need a marketing authorization from the TMDA. The marketing authorization is issued with a Marketing Authorization (MA) number which is included on the registration certificate.
Individuals or companies requiring further information on the procedures for licensing medicinal product in Tanzania should review the relevant documents, including guidelines and forms. You can also contact us at medicines@tmda.go.tz
Details of medicines registration guidelines which are applied during new marketing authorization applications, variations and renewals are available on the Links to guidelines.
Application Pathways
There are several different application pathways that a company may use to obtain a marketing authorization for a medicinal product depending on the discretion of the company.
National Procedure
A company can submit an application for a marketing authorization directly to the TMDA if the company only wishes to market a medicine in Tanzania. The procedure for marketing authorization of medicinal product in Tanzania are described below: -
New Products
Authorizations to market new medicinal products are granted by the TMDA under the Tanzania Medicines and Medical Devices (Registration of Medicinal Products) Regulations, 2015. Authorization for a medicinal product is called marketing authorization. Details on the content of an application are outlined in the Registration of Medicinal Products Regulations and in product specific guidelines all of which are available on the The TMDA website (link to the guidelines). All applications have to be submitted in accordance with the CTD (Common Technical Document) format. Details on the format of the dossier are outlined in respective product guidelines
Process flow
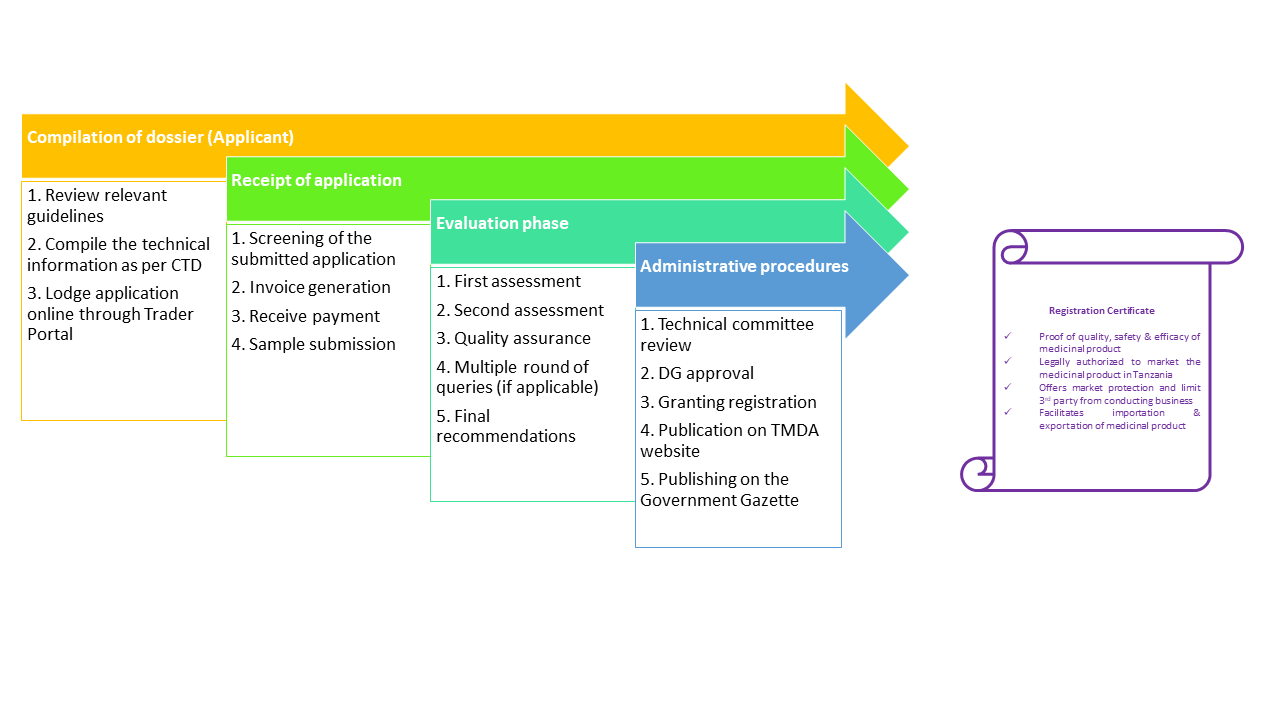
Authorization of Variations
Information for applicants on the process and requirements for notification and approval of changes to the terms of a marketing authorization.
After a medicine has been authorised, the terms of the marketing authorization may subsequently be varied. The procedures around such variations are governed by Tanzania Medicines and Medical Devices (Registration of Medicinal Products) Regulations and in product specific variation guidelines all of which are available on the The TMDA website. Tanzania Medicines and Medical Devices (Registration of Medicinal Products) Regulations, 2015 extends the scope of the variation’s regulation to all marketing authorizations, human, herbal and veterinary, granted marketing authorization. Detailed procedural guidance on the classification, submission and processing of variations has been published by the product specific variation guideline
Process flow
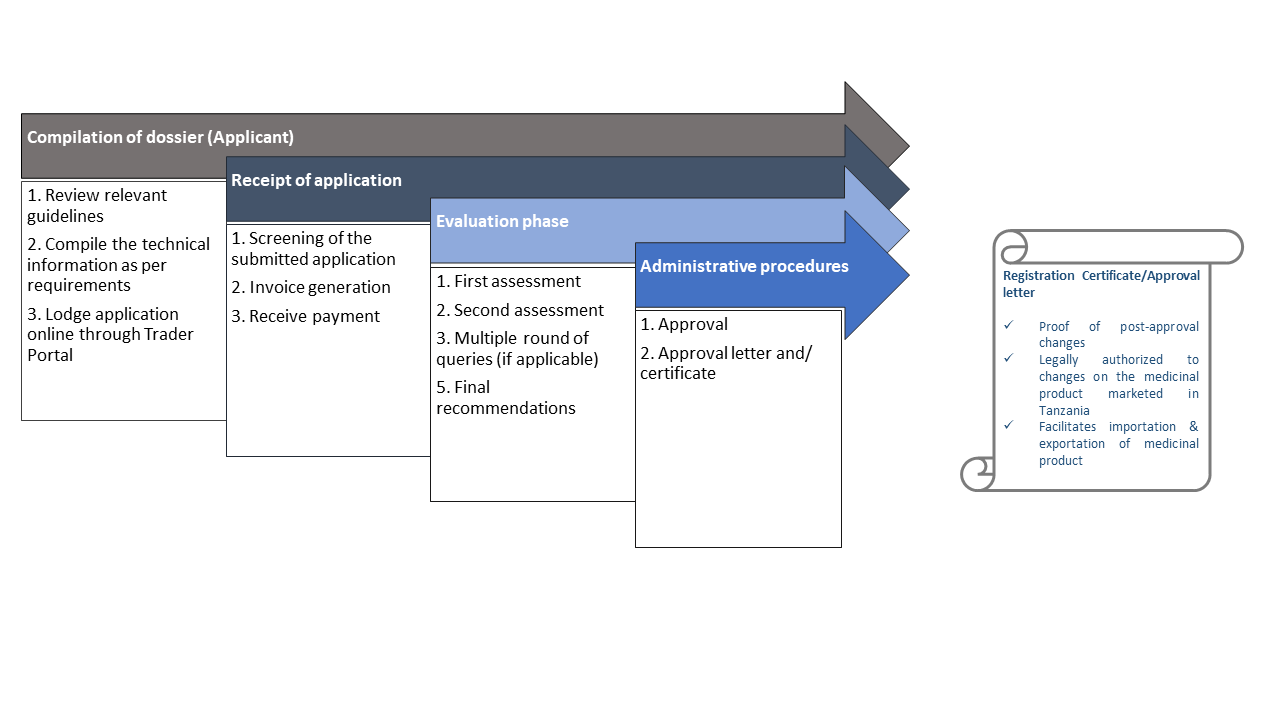
Renewal applications
Marketing authorizations are valid for five years from the date of first issue. For the authorization to remain valid, it should be renewed at the end of this five year period.
Renewal applications should be submitted to the TMDA at least three (3) months before the expiry of the authorization, although earlier renewals are acceptable in order to facilitate a common renewal date for a range of products. Renewal applications should be accompanied by the Application form for renewal of a marketing authorization.
For more information on preparing renewal applications, please refer product specific guidelines all of which are available on the The TMDA website
Process flow
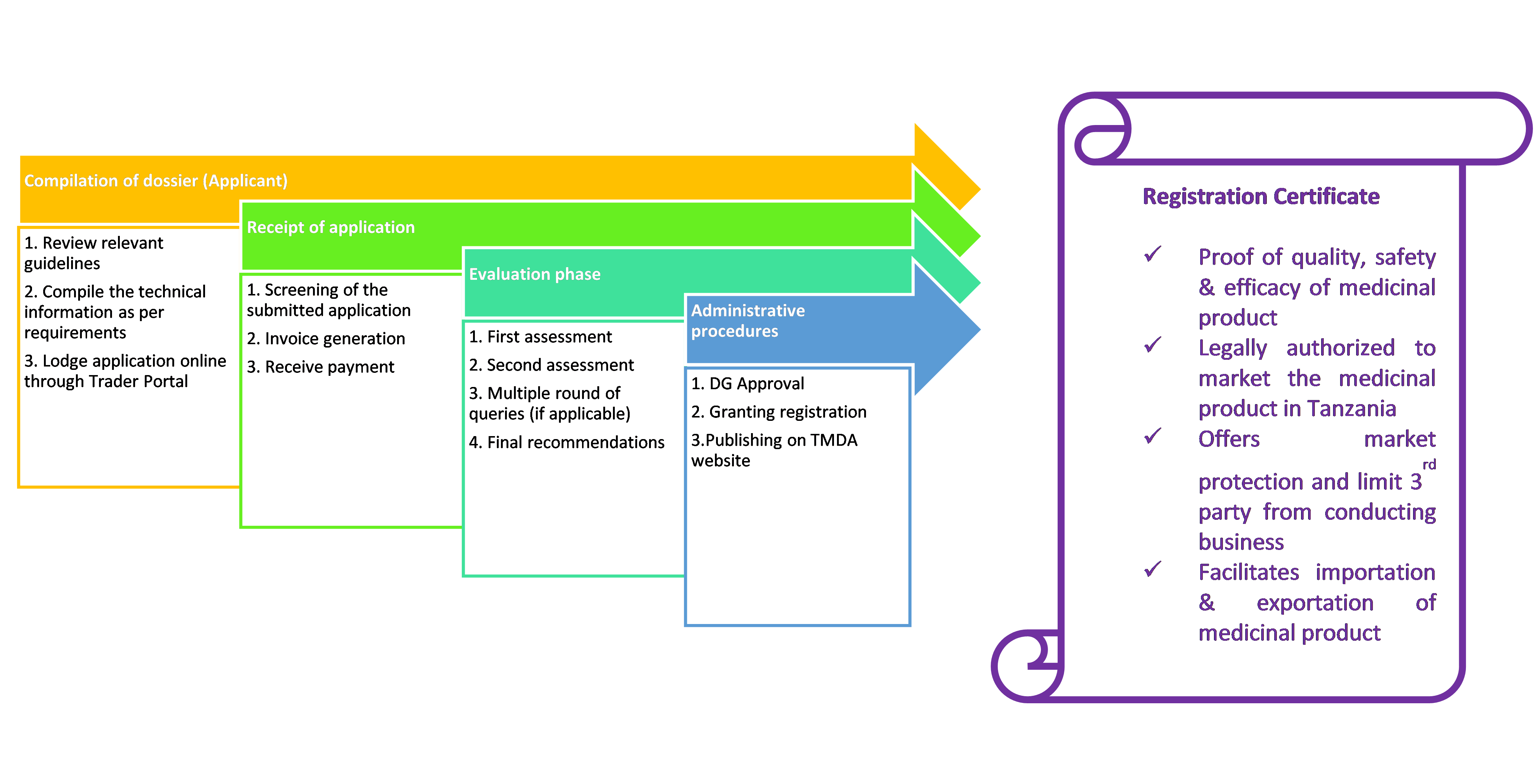
Withdrawal of marketing authorization
Withdrawal of an authorization / certificate may occur during the period of validity of the authorization or certificate or on renewal when the holder may decide not to renew it.
Withdrawal of an authorization or certificate may occur during the period of validity of the authorization or certificate or during the renewal when marketing authorization holder (MAH) decide not to renew it or any reason. In each case, the Authority should be notified of the intention to withdraw.
-
Voluntary withdrawal (withdrawal initiated by marketing authorization holder) Holder must
Holder must initiate the withdrawal of authorization through TMDA trader portal by attaching an official letter which should indicate the reason for withdrawal, which can be commercial or may relate to concerns regarding the quality (including GMP status), safety or efficacy of the product.
-
Mandatory withdrawal (withdrawal initiated by the Authority)
Withdrawal of an authorization or certificate may occur during the period of validity of the authorization or certificate when the MAH contravened the regulations or any provision of the Act or any reason relate to concerns regarding the quality (including GMP status), safety or efficacy of the product the registration of the products shall be withdrawn, suspended or revoked.
Fees and charges
| New Products | |||
| S/N | Product category | Product type | Fees |
| 1 | Domestic manufactured | Human and veterinary medicines | 1,000,000 TZS |
| Antiseptic and disinfectant | 100,000 TZS | ||
| 2 | Imported product | Human and veterinary medicines | 2,000 USD |
| Antiseptic and disinfectant | 300 USD | ||
| Biologicals | 3,000 USD | ||
| Authorization of Variations | |||
| S/N | Product category | Product type | Fess |
| 1 | Domestic manufactured | Major Variation | 200,000 TZS |
| Minor Variation | 100,000 TZS | ||
| 2 | Imported product | Major Variation | 1000 USD |
| Minor Variation | 300 USD | ||
| Renewal Applicaton | |||
| S/N | Product category | Product type | Fess |
| 1 | Domestic manufactured | Human and veterinary medicines | 1,000,000 TZS |
| Antiseptic and disinfectant | 100,000 TZS | ||
| 2 | Imported product | Human and veterinary medicines | 2,000 USD |
| Antiseptic and disinfectant | 300 USD | ||
| Biologicals | 3,000 USD | ||
Timeline
| S/N | Application | Product type | Process | Timeline |
| 1 | New Human and Veterinary Medicines | Domestic manufactured | Evaluation of medicinal products including vaccines | Within 60 days |
| upon receipt of completed application | ||||
| Evaluation of query responses | Within 30 days | |||
| Imported products | Evaluation of medicinal products including vaccines | Within 180 days | ||
| upon receipt of completed application from foreign | ||||
| manufacturer | ||||
| Evaluation of query responses | Within 80 days | |||
| Priority product (EAC, SADC, WHO, orphan medicines) | Evaluation of medicinal products including vaccines | Within 90 days | ||
| upon receipt of completed application | ||||
| 2 | New antiseptics and disinfectants | Domestic manufactured | Evaluation of product upon receipt of completed application | Within 30 days |
| Imported products | Evaluation of product upon receipt of completed application | Within 60 days | ||
| 3 | New Herbal Medicines | Domestic manufactured | Evaluation of product upon receipt of completed application | Within 90 days |
| Imported products | Evaluation of product upon receipt of completed application | Within 180 days | ||
| 4 | Approval of variations | Major variation of a registered medicine | Evaluation of product upon receipt of completed application | Within 45 days |
| Minor variation of a registered medicines | Evaluation of product upon receipt of completed application | Within 30 days | ||
| 5 | Renewal of marketing authorization | Domestic manufactured medicinal products | Evaluation of product upon receipt of completed application | Within 20 days |
| Imported products medicinal products | Evaluation of product upon receipt of completed application | Within 30 days | ||
Harmonization and collaborative procedures
A company can submit an application for a marketing authorization to TMDA and other Regional Economic Communities (RECs) member states if the company wishes to market a medicine within the region. For more detailed information please refer to the pathways: -

