Unregistered Products
Pursuant to Section 17 of TMDA Act, Cap 219 prohibit the manufacture, importation, exportation, sale, offering for sale, distribution, use or expose of drugs without the proper authorization.
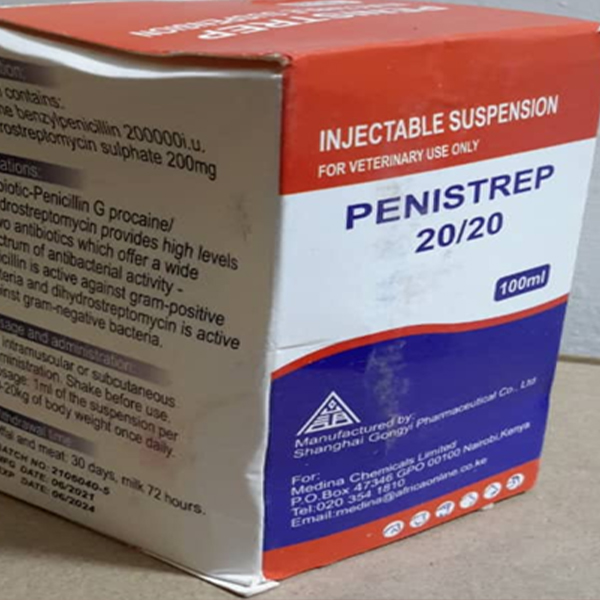
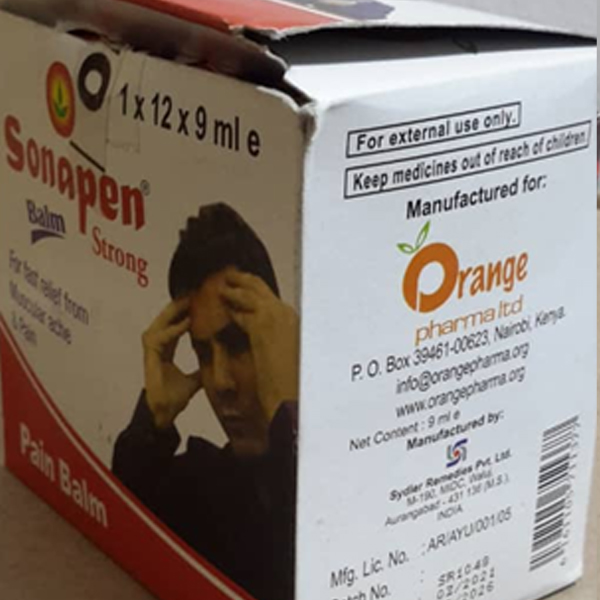
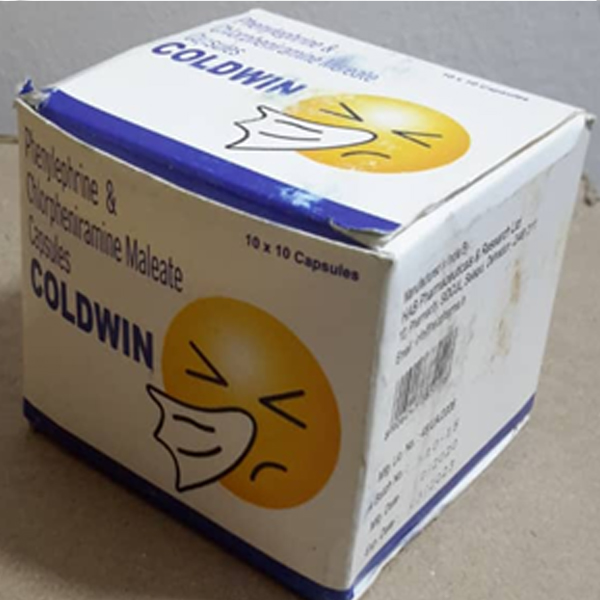
Through market surveillance TMDA has identified unregistered medicinal products circulating in the Tanzania market contrary to the legal requirements. The Authority warns the public from purchasing and consuming any unregistered medicinal product.
Moreover, All concerned establishments are warned not to distribute these products until they have been issued the appropriate marketing authorization (registration).
The unregistered medicinal products have not gone through the registration process therefore their quality, safety and efficacy is unknown. The use of unregistered products may pose health risks to consumers.
The list of registered products can also be accessed through the website (https://www.tmda.go.tz). To report any sale or distribution of unregistered medicinal products or enquiries contact TMDA though e-mail info@tmda.go.tz, call +255 22 2450512 / 2450751 / 2452108 or Toll free: 0800110084