Southern African Development Community – MRH project
Harmonization efforts in the Southern African Development Community (SADC) region are facilitated by the Southern African Regional Programme Access to Medicines and Diagnostics (SARPAM). SARPAM was initiated as a support programme for the SADC Pharmaceutical Business Plan, through the Department for International Development (DFID-UK) between 2009 and 2014.
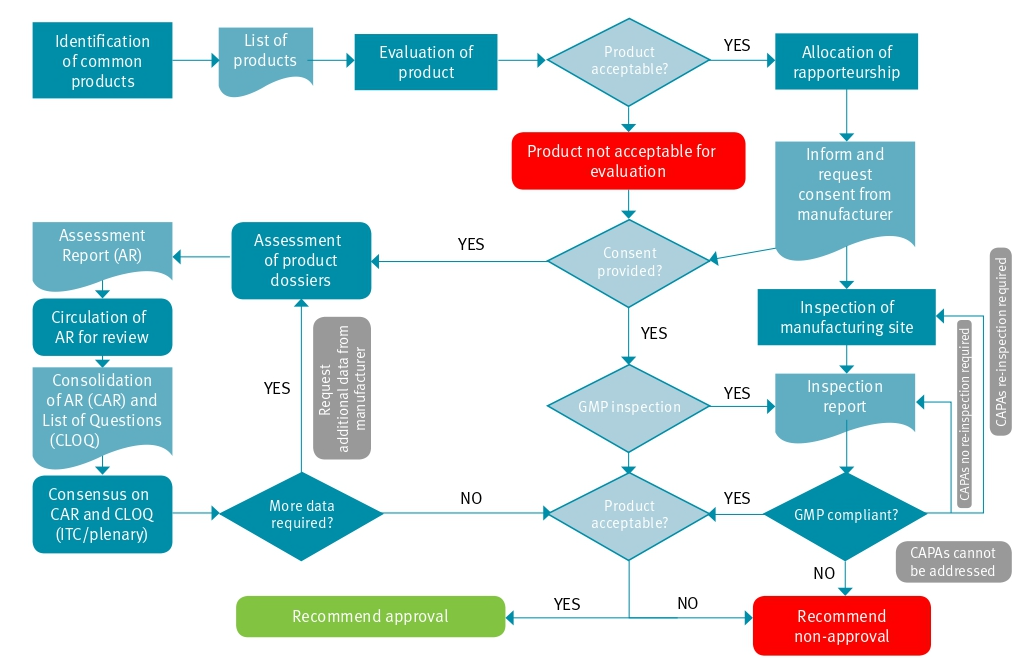
The SADC regional medicines registration is implemented through the ZAZIBONA project, which was initiated in 2013 and established as a collaborative procedure for medicines registrations between four SADC countries. In 2014, the ZAZIBONA approach was officially adopted as part of the broader SADC Framework for Regulatory Harmonization.
The Programme is implemented by National Medicines Regulatory Authorities (NMRAs) in SADC Partner States: Medicines Control Authority of Zimbabwe (MCAZ), The Botswana Medicines Regulatory Authority (BoMRA), Zambia Medicines Regulatory Authority (ZAMRA), The Namibia Medicines Regulatory Council (NMRC), South African Health Products Regulatory Authority (SAHPRA), Agence Congolaise de Réglémentation Pharmaceutique (ACOREP) of the Democratic Republic of Congo, Autoridade Nacional Reguladora de Medicamentos (ANARME) of Mozambique, Tanzania Medicines and Medical Devices Authority (TMDA), Pharmacy and Medicines Regulatory Authority (PMRA) of Malawi, Ministry of Health Eswatini, Agence du Médicament de Madagascar (AGMED) of Madagascar, Medicines Regulation Unit, Public Health Authority, Ministry of Health Seychelles, Ministry of Health of Lesotho, Direction des Laboratoires et des Pharmacies of Comoros.
SADC – MRH (ZAZIBONA) Process Flow