Medical Devices and Diagnostics Control – Section Profile

Sunday Kisoma
Manager, Medical Devices and Diagnostics Control
Medical Devices and Diagnostics Control Section performs the following functions in regulation of medical devices, in vitro diagnostics and oxygen for medical use in fulfillment of the Authority’s mission of protecting and promoting public health: -
- Evaluation and Registration of medical devices and diagnostics,
- Licensing and Inspection of premises dealing with business of medical devices and diagnostics,
- Licensing and Inspection of Manufacturers of medical devices and diagnostics,
- Assessments and Issuance of permits for importation and exportation of medical devices and diagnostics,
- Post marketing surveillance for quality and performance of the devices and diagnostics circulation on the market,
- Monitoring and assessment of adverse events and quality defects associated with the use of medical devices and diagnostics,
- Quality Audits of domestic and overseas manufacturing facilities,
- Control of Promotional promotions and advertisement of medical devices and diagnostics.
- Lot to lot laboratory testing of critical medical devices and diagnostics,
- Development of regulations, guidelines and technical standards for medical devices and diagnostics used in the country,
- Regulatory control of oxygen for medical use, including licensing and inspection of manufacturers, dealers and hospitals.
Introduction
Medical Devices and Diagnostics Control is a section under the Directorate of Medical Products Control, which deals with regulation of Medical Devices, In Vitro diagnostics and Medical Oxygen by carrying out assessment and registration, post marketing surveillance, vigilance, licensing and inspection activities.
According to the Tanzania Medicines and Medical Devices Act, Cap 219 and the Tanzania Medicines and Medical Devices (Control of Medical Devices) Regulations, 2015; a medicinal device means an instrument, apparatus, implement, medical equipment, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part or accessory, which is -
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease, in man or other animals or;
- intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its principal intended purposes through chemical action within the body of man or other animals and which is not dependent upon being metabolised for the achievement of any of its principle intended purposes.
Personnel
The Section is headed by the Manager leads the team of sixteen (16) full time Officers and Senior Officers. The team is made of qualified and experienced experts from the fields of pharmacy, biomedical engineering, microbiology, medical laboratory sciences, chemistry and electronics
Product Life Cycle management
After the medical device or diagnostic is granted marketing authorization by the Authority, it remains the responsibility of the registrant to ensure that the product continuously maintains its quality, safety and performance profile during its life cycle. During this time registrants are required to file in for any variations on the products and premises which may have implication on quality of the products. In addition, TMDA continuously monitor these attributes through vigilance and post marketing surveillance activities. Marketing authorization for all medical devices and diagnostics remains valid for a maximum period of five years, after which the products will require renewal of registration
list of notified medical devices and diagnostics
list of registered medical devices and diagnostics
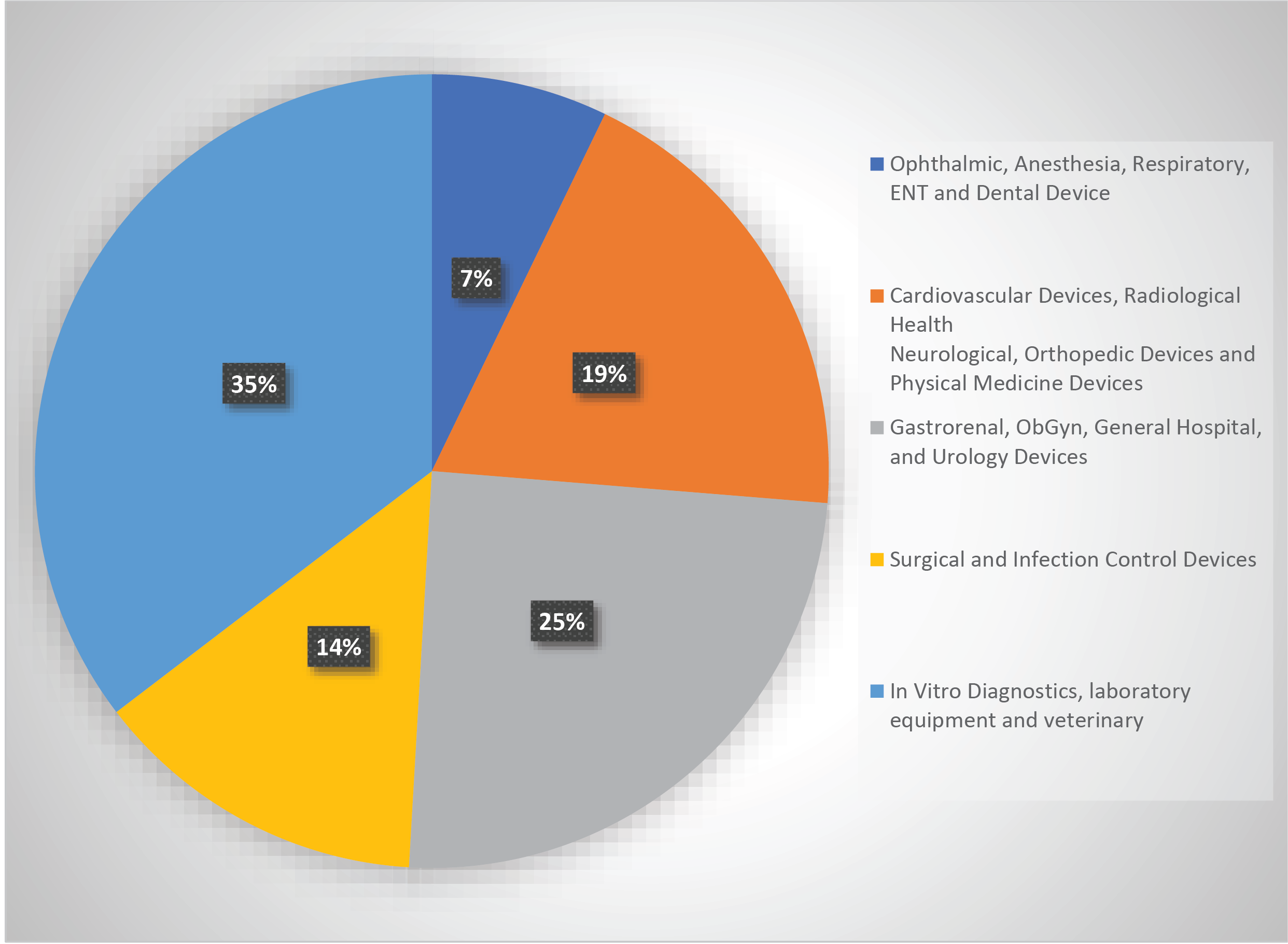
Proportions of approved products