East African Community – MRH project
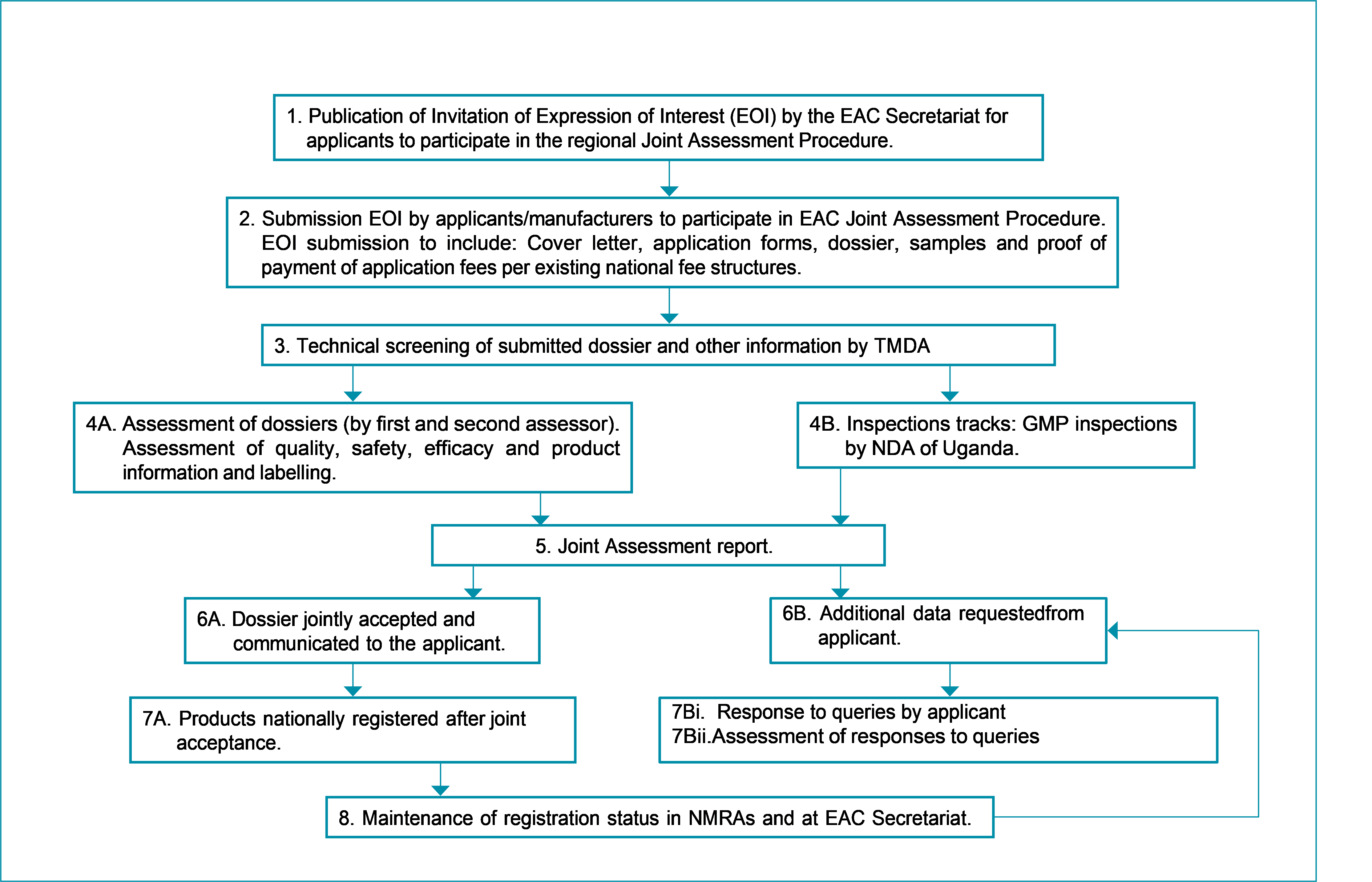
The AMRH programme selected the EAC as the first region to begin implementation of its regulatory harmonization plan and officially launched on 30 March 2012. The programme aimed to implement harmonized technical requirements, information management systems and quality management systems in each EAC Member State and to build regional and national capacity to implement an EAC-MRH programme. The programme was also initiated to create a platform for information sharing and to develop and implement a framework for mutual recognition of regulatory decisions. In September 2014, the EAC-MRH finalized and approved harmonized registration guidelines, the common technical dossier (CTD), GMP and the quality management system (QMS) compendia. These harmonized guidelines were launched in January 2015 and have been used for several national registrations as well as EAC joint dossier assessments.
The Programme is implemented by National Medicines Regulatory Authorities (NMRAs) in all EAC Partner States: The Department of Pharmacy, Medicines and Laboratories (DPML) of Burundi, The National Drug Authority (NDA) of Uganda, Pharmacy and Poisons Board (PPB) of Kenya, Rwanda FDA, Drug and Food Control Authority (DFCA) of South Sudan as well as The Tanzania Medicines and Medical Devices Authority (TMDA) and The Zanzibar Food and Drugs Agency (ZFDA) of the United Republic of Tanzania.
Specific objectives
- To implement an agreed upon common technical document for registration of medicines in the EAC Partner States
- To implement a common information management system for medicines registration in each of the EAC Partner States’ NMRAs which are linked in all Partner States and EAC Secretariat
- To implement a quality management system in each of the EAC Partner States’ NMRAs
- To build regional and national capacity to implement medicines registration harmonization in the EAC
- To develop and implement a framework for mutual recognition based on Chapter 21, Article 118 of the East African Community Treaty
- To create a platform for information sharing on the harmonized medicines registration system to key stakeholders at national and regional level
EAC Joint Procedure