Latest News
|
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF HEALTH TANZANIA MEDICINES AND MEDICAL DEVICES AUTHORITY |
|
ISO 9001: 2015: CERTIFIED
PUBLIC NOTICE
02nd May, 2022
TMDA AUTHORIZES NOVEL ORAL POLIO VACCINE TYPE 2 (nOPV2) VACCINE FOR EMERGENCY USE DURING OUTBREAK OF POLIOMYELITIS VIRUS TYPE 2.
- Tanzania Medicines and Medical Devices Authority (TMDA) has authorized for emergence use of Polio Type 2 (nOPV2) vaccine. The authority is issuing this notice pursuant to section 57-(1) of The Tanzania Medicines and Medical Devices Act, Cap 219 of 2019 that empower the Authority to authorize the use of unregistered medicinal products including vaccines for specific purpose.The Ministry of Health has declared nOPV2 vaccine manufactured by PT Bio Farma (Persero), Indonesia to be used during the outbreak of Poliomyelitis Virus Type 2 when is required by Global Polio Eradication Initiative (GPEI) or World Health Organization (WHO).
- This authorization was based on the safety, quality and efficacy data submitted by applicant through World Health Organization (WHO) Emergency Use Listing (EUL) Procedure. Emergency Use Listing Procedure (EUL) is a risk-based procedure for assessing and listing unapproved vaccines with the aim of expediting the availability of this product during a declared state of public health emergency.
- Furthermore, the Authority would like to highlight to the public that, granting of emergency use of this vaccine does not alter the standard regulatory requirements for establishing quality, safety and efficacy required for issuing marketing authorization to the designated products.
- The summary information of nOPV2 vaccines approved by the authority can be accessed by clicking here
SUMMARY INFORMATION OF NOVEL ORAL POLIOMYELITIS VACCINE TYPE 2 (nOPV2)
1.1
Trade name
Novel Oral Poliomyelitis Vaccine Type 2(nOPV2)
1.2
Generic name
Live attenuated novel poliomyelitis virus type 2
1.3
Strength of immunogenic substance(s):
Live attenuated novel poliomyelitis virustype 2 ≥ 105.0 CCID50.
1.4
Dosage form
Clear suspension for oral use.
1.5
Distribution category
POM
1.6
Primary Packing materials /Pack size
Type 1 USP grass vial of 5 mL.
1.7
Secondary Packing materials/Pack size
10 x 5 mL vial in a carton box
1.8
Route of Administration
Oral
1.9
Storage condition
nOPV2 stored for up to 36 months when frozen at ≤ - 20˚C, with up to 6 months at 2-8˚C allowed
1.10
Proposed Shelf life
36 months when frozen at ≤ - 20˚C, with up to 6 months at 2-8˚C allowed
1.11
Visual Description of finished product as finished product specifications
A clear virus suspension, slightly yellow to light red colour.
1.12
Applicant
PT Bio Farma (Persero)
Jl. Pasteur No. 28
Bandung 40161
Indonesia.
1.13
Immunogenic substance manufacturer
PT Bio Farma (Persero)
Jl. Pasteur No. 28
Bandung 40161
Indonesia
1.14
Manufacturers
PT Bio Farma (Persero)
Jl. Pasteur No. 28
Bandung 40161
Indonesia.
1.15
Country of origin
Indonesia
1.16
Therapeutic Indication(s):
Active immunization in all age groups for emergency use in response to outbreaks caused by type 2 poliomyelitis virus when and where it is required by the Global Polio Eradication Initiative (GPEI) or World Health Organization (WHO).
Product Photograph:
Close Issued by: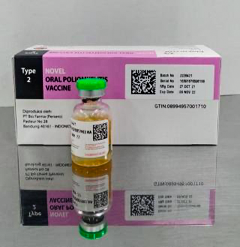
Director General,
Tanzania Medicines and Medical Devices Authority (TMDA),
P. O. Box 1253, Dodoma Or
P. O. Box 77150, Dar es Salaam
Phone: +255 22 2452108/2450512/2450751,Fax: +255 22 2450793



