Latest News
Posted On: Sep 29, 2020
TANZANIA MEDICINES AND MEDICAL DEVICES AUTHORITY

ISO 9001: 2015 CERTIFIED
PUBLIC NOTICE
24th September, 2020
CLARIFICATION ON THE INFORMATION SPREADING VIA SOCIAL MEDIA CONCERNING THE QUALITY AND SAFETY OF MEDICINAL PRODUCTS CONTAINING THE ACTIVE PHARMACEUTICAL INGREDIENT; REMDESIVIR USED FOR THE TREATEMENT OF COVID-19
- Recently there has been information, spreading on social media regarding the medicinal products containing the active ingredient “Remdesivir” which is currently recommended for treatment of COVID-19.
- The medicines containing the aforementioned active ingredient are known by the brand names “Covifor “and “Jubi-R” manufactured in India by Hetero Labs Limited and Jubilant Generics Limited respectively.
- These injectable products are in packages whose labels indicate that they are not allowed to be distributed in the United States, Canada and the European Union.
- The statement “Not for distribution in US, Canada and EU” on the packaging labels (see picture below) have raised concerns as to whether or not the medicines distributed in other parts of the world are unsafe or sub-standard, and unsuitable for human consumption.
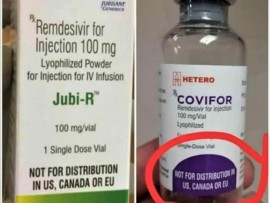
- Tanzania Medicines and Medical Devices Authority (TMDA) would like to clarify that; the use of such words in labeling is a common practice in the pharmaceutical sector and aims to prevent the distribution of medicines in the United States, Canada and the European Union where Remdesivir has been granted patent.
- Redemsivir was first discovered by Gilead Limited which is a company based in the United States of America and the product is currently authorized for use by the Food and Drug Administration (US-FDA) for the treatment of COVID-19 disease under Emergency Use Authorization (EUA) procedure.
- This procedure is used by the US-FDA for the approval of medicines which are still undergoing clinical trials and awaiting official approval for human use in order to treat or prevent the rapid and highly contagious diseases with no alternative medication such as COVID-19, Ebola and others.
- In this respect, Covifor and Jubi-R are manufactured by the two Indian companies after being granted special permit by Gilead Limited to manufacture generic versions so they can be distributed to other countries (a total of 127 thus far) globally in accordance with the agreement excluding the United States, Canada and countries in the European Union.
- Upon expiration of the patent; which is usually between 10 to 20 years depending on the Intellectual Property Rights and laws of the respective countries, other companies will partake in the marketing and distribution of this drug for the treatment of COVID-19.
- Nevertheless, the medicinal products named Covifor and Jubi-R are not yet registered with TMDA for use in the country.
- For more information, please contact us through our Headquarters’ Offices or Zone Offices located in Dodoma, Mwanza, Tabora, Mbeya, Arusha, Mtwara and Dar es Salaam.
Acting Director General,
Tanzania Medicines and Medical Devices Authority (TMDA),
PSSSF House, 10th Floor,Makole Street,
P. O. Box 1253, Dodoma Or
P. O. Box 77150, Dar es Salaam
Phone: +255 22 2452108/2450512/2450751
Fax: +255 22 2450793
Toll free: 0800110084
Website: www.tmda.go.tz

