

As we look forward in ensuring quick and effient services to cutomers, we have much utilized to electronic systems that play a big role in implementing Client service charter. Information and services provided over the Internet are as follows below
A: PAPER BASED MEANS OF REPORTING
Mail:
Tanzania Medicines and Medical Devices Authority,
P.O. Box 1253, Dodoma
P.O. Box 77150, Dar es Salaam, Tanzania.
Fax: +255 22 2450793
Email: clinicaltrials@tmda.go.tz
Choose any of the following convenient ways to report ADR/AEFI
Browse sqrt.tmda.go.tz from your computer or mobile phone

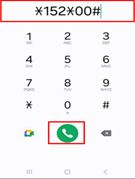
Dial *152*00# on your mobile phone

Install the SQRT App on your mobile phone

As we look forward in ensuring quick and effient services to cutomers, we have much utilized to electronic systems that play a big role in implementing Client service charter. Information and services provided over the Internet are as follows below
One of the means for ensuring that medicinal products meet the required standards of quality, safety and efficacy is by conducting product specific pre-marketing assessments to determine whether the product should be registered.


You can also send complements, complaints or enquiries through the government portal that will make follow-up on about your comments so as to increase public satisfactory.
Please click here, to visit the portal